Dive Decompression Theory
In 1906, John Scott Haldane was commissioned by the Royal Navy to study Dive Decompression Theory and solve the problem of divers getting DCS. Because of Dive Decompression Theory now a century later virtually all dive computers and dive tables in use today are derived from the Dive Decompression Theory theoretical model that he invented. He drew on the work of Paul Bert, a French physiologist who had identified the cause of DCS a few decades earlier.
Dive Decompression Theory by Paul Bert (1833 – 1886)
Paul Bert is remembered mostly for his Decompression Theory classical work, La Pression barometrique (1878), which laid the foundation of knowledge of the physiological effects of air-pressure, both above and below atmospheric pressure. Bert became interested in the problems that low air pressure caused for mountain climbers and balloonists. This led him to study the problems that divers had with increased pressure as well.
Bert’s research and experiments led to his conclusion that pressure does not affect the body physically, but rather chemically, by changing the proportions of oxygen in the blood. Too little creates oxygen deprivation and too much creates oxygen poisoning. He showed that pure oxygen under high pressure can be deadly and to this day Central Nervous System (CNS) oxygen toxicity (discussed in physiology) is known as the ‘Paul Bert Effect’.
Perhaps his most important discovery was the effects of nitrogen under high pressure, which for the first time explained decompression. In investigating Dive Decompression Theory and the causes of decompression illness Bert exposed 24 dogs to pressure of 7-9 ¾ atmospheres (equivalent to a depth of 87.5msw) and decompressed them rapidly in 1-4 minutes. The result was that 21 died, while only one showed no symptoms. In one of his cases, in which the apparatus burst while at a pressure of 9 ½ atmospheres, death was instantaneous and the body was enormously distended. However, he also found that dogs exposed, for moderate periods, to similar pressures suffered no ill effects provided that the pressure was relieved gradually, in 1-1 ¾ hours.
He determined that the symptoms observed were due to the formation of gas bubbles in the blood and tissues. He also identified nitrogen as the gas which was producing the bubbles. He went on to explain that it was the increase in partial pressure of nitrogen which caused nitrogen to become dissolved in the bodies tissues and then the subsequent reduction in pressure caused the nitrogen to come out of solution and form bubbles. As a result of this research Bert concluded that divers decompress slowly and at a constant rate “for they must not only allow time for the nitrogen of the blood to escape but also to allow the nitrogen of the tissues time to pass into the blood”.
He also went on to suggest stopping divers halfway to the surface during decompression after a deep dive and as such was the first to suggest what are now known as deep stops.
Bert carried out a number of Dive Decompression Theory experiments into methods of treating the compressed air illness once the symptoms had appeared. His experiments showed that once bent, the symptoms could be relieved by returning into the compressed air environment and then decompressing the patient slowly. He also showed that breathing pure oxygen was highly effective in relieving the symptoms of decompression illness. In one of his experiments on animals he noted: “The favorable action of oxygen was . . . evident; after several inhalations (of oxygen) the distressing symptoms disappeared.”
In a later entry, Bert attempted to explain why oxygen worked. “I thought that if the subject were caused to breathe a gas containing no nitrogen — pure oxygen for example — the diffusion would take place much more rapidly and perhaps would even be rapid enough to cause all the gas (nitrogen) to disappear from the blood.” This is indeed why oxygen is so useful in treating decompression illness. Bert was the first to propose the concept of oxygen recompression therapy in his Dive Decompression Theory, though the actual practice wasn’t implemented until many years later.
John Scott Haldane (1860-1936) Father of Modern Dive Decompression Theory
Scottish physiologist John Scott Haldane is considered to be the father of modern dive decompression theory. Haldane was the first scientist to apply a scientific approach to predicting and preventing decompression in a systemized way.
In 1906, Oxford physiologist Haldane discovered that the respiratory reflex is triggered by an excess of carbon dioxide in the blood rather than a lack of oxygen (discussed in physiology).
It is Haldane’s work on decompression for which he is most widely remembered, especially amongst divers. In 1906 Haldane was approached by the Royal Navy’s Deep Diving Committee to carry out research on a number of aspects of their diving operations. The most important aspect of this work was looking at ways to avoid the ‘bends’ or “caissons disease” as it was then widely known.
It had long been observed that men working in pressurized bridge and tunnel construction areas, known as caissons, would sometimes complain of pain in their joints. As the depth they were working at increased, and so the pressure inside the caisson also increased, the severity of the symptoms increased. Many suffered total paralysis and there were frequent deaths. Research in this decompression theory and practical observation suggested that gasses, breathed under pressure by the workers, were diffusing into the body’s tissues and when these gasses came out, in the form of bubbles in the body, the workers got caisson disease, or what we now call decompression sickness (DCS).
The same symptoms were seen amongst divers who were breathing air under pressure. Divers were told to minimize this risk by ascending slowly to begin with, and then rising faster as they got nearer the surface. Thanks to Haldane’s dive decompression theory work, we know now that this was incorrect and potentially dangerous.
Haldane began experimenting on goats as they were readily available subjects and of a similar size to humans. He found that the body could tolerate a certain amount of excess gas with no apparent ill effects. Caisson workers pressurized at two atmospheres (10 msw) experienced no problems, no matter how long they worked. Similarly goats saturated to 50 msw did not develop DCS if decompressed to half ambient pressure.
In order to explain his observations, Haldane suggested in his dive decompression theory that we consider the body as a group of tissues which absorbed and released gases at different rates. This meant the tissues were all exposed simultaneously to the breathing gasses at ambient pressure, but each tissue reacted to the gas pressure in a different way. He then went on to suggest a mathematical model to describe how each of the tissues absorbs and releases gases and put limits on the amount of over pressurization that the tissues could tolerate.
In his dive decompression theory Haldane introduced the concept of half times (discussed more later) to model the uptake and release of nitrogen into the blood. The half time is the time required for a particular tissue to become half saturated with a gas. He suggested 5 tissue compartments with half times of 5, 10, 20, 40 and 75 minutes. Haldane also developed practical dive tables based on his research that included slower ascent rates as the diver approached the surface.
Following the report of the Admiralty’s Deep Diving Committee it was decided to publish the committee’s conclusions about this dive decompression theory in the form of a blue book available to the public. The conclusions were universally accepted and it became the foundation of all diving operations, both in the UK and abroad. In 1912 the US Navy adopted the tables and these tables were used by all US Navy divers up until 1956.
Haldanean Model
The Haldanean model consists of multiple theoretical tissue compartments (discussed more later). Gas always goes from a higher pressure to a lower pressure until equilibrated.
When a diver descends, the nitrogen pressure in breathing air is higher than the pressure of nitrogen dissolved in the body. Consequently the nitrogen dissolves into the body’s tissues and continues to do so until the pressure is equilibrated. These body tissues are represented in the model by theoretical tissue compartments.
When a diver ascends, it is now the pressure of nitrogen that is dissolved in the body that is higher than the nitrogen pressure in breathing air. And so, the nitrogen dissolves out of the body’s tissues, into the bloodstream, and is then exhaled during breathing.
The pressure gradient is the difference between the pressure of nitrogen dissolved in the body and the surrounding pressure. As long as the ascent is slow, the pressure gradient is kept well within limits and the nitrogen in solution within the body dissolves harmlessly out of the body.
If the ascent is too fast, the pressure gradient is too great, that is the difference in pressure of nitrogen dissolved in the body and the surrounding pressure. The nitrogen comes out of solution faster than the body can eliminate it and bubbles form causing DCS. In dive decompression theory we learn to prevent this.
Haldane assumed that all bubbles result in DCS. However, we now know today that silent bubbles do not display symptoms of DCS.
You can rely on a Haldanean dive decompression model only as far as it has been shown to work in tests and by field experience. The relationship between the body and Haldanean dive decompression models is not direct, but implied based on actual dive data.
Doppler Ultrasound Flowmeter
The link with diving medicine comes from the ability of Doppler ultrasound technology to detect bubbles moving in blood vessels and so to detect the presence of bubbles in those vessels after diving.
Decompression from most dives causes a degree of bubble formation in the veins. It is generally accepted that the numbers of these bubbles are an indicator of the probability of decompression sickness (DCS) developing (higher numbers of bubbles are more likely to result in DCS). Silent bubbles and few of them, do not result in symptoms of DCS!
With regard to the significance of the bubbles themselves, there are a few problems. Bubbles are commonly detected in the veins following dives that do not produce DCS. While the risk of developing DCS does appear to be greater following those dives that produce high bubble grades, a significant proportion of such dives still do not result in obvious problems. It follows that Doppler bubble detection is certainly not a valid diagnostic test for DCS, and high bubble grades on Doppler in the absence of DCS symptoms would not be an indication for recompression treatment.
Tissue compartments
The body various tissues absorb and release nitrogen at different rates. To account for this his dive decompression theory, Haldane constructed a mathematical model consisting of multiple theoretical compartments.
His original decompression model consisted of five tissues/compartments. The US Navy tables later revised this to 6 compartments, whilst the Recreational Dive Planner (which we use today) adapted this number to 14 to better suit the profile of recreational divers (discussed more later).
Theoretical tissue compartments do not represent any particular body tissues, and are simply part of a mathematical model, accounting for the fact that the body doesn’t absorb and release nitrogen on a singular basis. This will always be part of a PADI IDC exam and so it is important to learn this dive decompression theory.
Nitrogen loading and Halftimes
If a diver hasn’t been diving for a while, a decompression model will consider all of the compartments to have a nitrogen loading of zero. If a diver were to dive to 30m for example, according to Haldane’s dive decompression theory model, all of the compartments will absorb nitrogen, and given enough time, will eventually saturate and reach equilibrium to nitrogen loading of 30m.
By applying halftimes, we can predict how long it will take for each compartment to reach a specific nitrogen loading for a given dive time. Halftimes are based on mathematical tables.
As we know, gas always goes from a higher pressure to lower pressure until equilibrated. Hence, nitrogen dissolves into and out of the body tissues until the pressure of nitrogen dissolved in the tissues is the same as the surrounding pressure. And so, the halftime of a compartment is the time it takes for the compartment to become half saturated, or if we are ascending to become half desaturated.
So if we consider a compartment with a half time of 5 minutes this means that compartment will become 50% saturated within 5 minutes. It will then take a further 5 minutes for the compartment to move from the current state to half way to saturation, i.e. 75%. The dive decompression theory table below shows the progression of the tissue saturation at 5 minute intervals.
|
5 min |
50% |
|
10 min |
75% |
|
15 min |
87.5% |
|
20 min |
93.75 |
|
25 min |
96.88% |
|
30 min |
98.44% |
We can see from this table, that the largest movement takes place during the first half time period, each subsequent half time period then sees a smaller and smaller change. If we draw a graph of gas uptake over time we can see a smooth line that initially shoots up but then gets ever shallower as it reaches 100%.

Mathematically the tissue will never reach 100% as it only moved half of the way from where it is towards 100% at each stage so it takes smaller and smaller steps towards its goal but always has the other half of the last step to cover. However, for practical purposes, after 6 periods we can consider the tissue saturated as it is at 98.44% saturation and after 24 hours we would consider the tissue to be completely saturated.
Each compartment in this dive decompression theory will saturate at a different rate. As we have seen after 5 minutes the 5 min tissue is 50% saturated but the 10 min tissue will take 10 minutes to become 50% saturated and so on. This means that each of the tissues will have a different level of saturation with the fast tissues absorbing gas and moving towards saturation faster than the slow tissues. However, as the diver ascends and the pressure is reduced the fast tissues will also release inert gas faster as the halftime also refers to the time it takes to release 50% of the absorbed gasses. This means that fast tissues will also release gases faster than slow tissues.
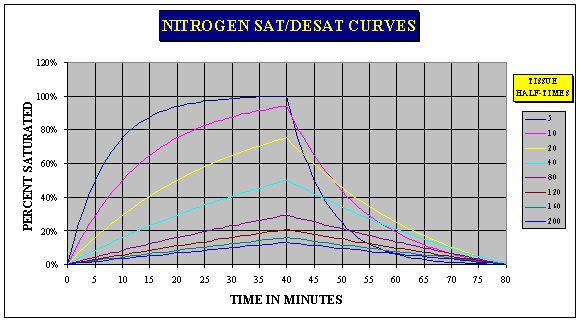
From the dive decompression theory graph above we can see the behavior of a number of compartments when considered together. As we have seen above the 5 min compartment is 50% saturated after 5 minutes, at 10 minutes it is 75 % saturated and so on until 30 minutes or 6 half-time periods it is effectively saturated (98.44%). The 10 min compartment is 50% saturated after 10 minutes. After 40 minutes the compartment is not yet saturated as this has only been 4 half time periods. It would require 6 half-time periods (60 minutes) for this compartment to become effectively saturated. The 40 min compartment is at 50% saturation at 40 minutes but all of the slower compartments; 80, 120 and 160 min are less than 50% saturated.
At 40 minutes the pressure is released and the compartments start to desaturate. The 5 min compartment was the most saturated but as it desaturates equally fast the level of saturation quickly drops to below that of the 10 min compartment and soon drops below the other compartments until at time 60 minutes, 20 minutes after the pressure was released, the 5 min compartment is less saturated than all of the other compartments. The 10 min compartment also drops quickly so that at 48 min it drops below that of the 20 min compartment.
It can be said that fast compartments are those with short halftimes and that the slow compartments are those with long halftimes.
M-values
M-values are based on test dives.
The “M” in M-value stands for “Maximum.” For a given ambient pressure, an M-value is defined as the maximum value of inert gas pressure that a hypothetical “tissue” compartment can “tolerate” without presenting overt symptoms of decompression sickness (DCS).
We can also say that the M-value is the maximum tissue pressure allowed in the compartment when the diver surfaces and that these maximum surfacing nitrogen levels in a model are determined by the M-value. If the diver exceeds the M-value of any compartment, the diver is at unacceptable risk of DCS.
Controlling Compartments
All compartments differ from on another within a model in two important ways. Firstly, they absorb nitrogen (inert gas) at different rates, which are characterized by halftimes. Secondly, they can tolerate different amounts of nitrogen (inert gas), which is the M-value, also known as allowable nitrogen loading.
It may help to think of each compartment filling a glass of water by a valve/tap. The 5-minute compartment has the largest (quickest) valve, so it fills the fastest. The 60-minute compartment has the smallest (slowest) valve and so it fills the slowest. All the glasses are the same size, and so each compartment will eventually fill the glass just at different rates.
On any given dive, the controlling compartment is the compartment that determines what the model makes a diver do. On a no-stop dive, it is the compartment that sets the no-stop limit by reaching its allowable limit (M-value) first.
The dive decompression theory M-value expresses the compartments maximum allowable pressure in meters of seawater (msw) or atmospheres (ata). Typically, at depths of around 30m it is the 5-minute compartment which controls the dive, that is to say it reaches its allowable limit first (allowable nitrogen limit). However, it’s at shallower depths that the slower compartments come into play.
For arguments sake, let’s say we are diving to 24m/ 3.4ata and that the maximum allowable limit of the 5-minute compartment is 30m/4ata, and that the maximum allowable limit of the 10-minute compartment is 24m/3.4ata. The 5-minute compartment can never reach its maximum allowable pressure of 30m or 4ata at this depth. At this depth it is the 10-minute compartment is the fastest compartment that can reach its allowable limit.
And so it is fair to say in dive decompression theory that faster compartments cannot control shallower dives. (see page 5-60 of the Encyclopaedia of Recreational Diving)
US NAVY Tables and Repetitive Diving
The US Navy tables were the industry standard in dive decompression theory until the mid-1980s. Based upon their research (during the 1950s) they made revisions to the tables previously developed by Haldane.
One change that they made was adding a compartment giving the model six compartments as opposed to Haldane’s five. Navy data showed that there were body areas with longer halftimes than Haldane’s 75minute longest compartment.
Designing for decompression diving, they reasoned that the worst case for any possible dive situation (e.g a repetitive no-decompression dive) was, if the slowest compartment, controlled the repetitive dive. Therefore, the US Navy tables and their surface interval credit are based on the elimination of nitrogen of the 120 minute compartment. This is why it takes 12 hours (720 minutes or 6 halftimes) before a dive is no longer considered a repetitive dive with the US Navy tables.
There are several reasons why the tables were at one time the dive decompression theory industry standard. Firstly, personal computers were not very common and developing a table was a tedious process. Second, many early divers and instructors came from the military and so adopted the US Navy tables as they were used to them. Third, the tables were easily available to the public.
Dr Raymond E Rogers and the RDP
During the early 1980’s, PADI Divemaster DR Rogers began examining the basis of the US Navy tables. He suspected that they were not ideal for recreational diving. He concluded 3 things:
120 minute halftime: Whilst appropriate for decompression diving, he noted that the 120 minute halftime for calculating washout/surface interval credit, might be overly conservative for exclusively recreational diving.
Test group: The test group used by the Navy, again whilst appropriate for military needs, didn’t represent the recreational diving population.
Conservatism: Doppler ultrasound flow metres found silent bubbles often formed on dives to the Navy limits, and a lower M-value might be more appropriate for non-military diving.
Hence, between the 120 minute compartment and its higher M-values, it was clear that the tables could be overly conservative or insufficiently conservative, depending on dive circumstances.
Following this dive decompression theory research, Rogers went onto develop the PADI Recreational Dive Planner – RDP to meet the needs specifically of recreational diving. The most significant decompression theory change to Rogers adaptation of Haldanes model, was the choice of a 60 minute halftime compartment as the basis of repetitive diving. As a result of this, the RDP gives about twice as much surface interval credit. This means the residual nitrogen time for a repetitive dive is roughly cut in half.
Example:
First dive: 18m for 30 minutes
Surface interval: 2:00
Residual Nitrogen time USN RDP
for a dive to 18m: 24 minutes 11 minutes
The RDP model also has 14 compartments with halftimes ranging from 5 minutes to 480 minutes. Roger implemented the WX and YZ rules to accommodate the fact that any compartment slower than 60 minutes could control a repetitive dive. The table also has lowered M-values.
The US Navy and other tables use different models to the RDP, and so pressure groups and letters are not interchangeable between the RDP and other tables. Letter designations represent different theoretical nitrogen levels.
Testing of the RDP covered a broader demographic range than the USN testing, with females, wider age range and differing physical types included.
Dive Computers
Dive computers are based on dive decompression theory and simply write a custom dive table for the exact depths, times and surface intervals of an individual, affording more dive time by eliminating unnecessary rounding.
Computers can be set into three basic groups:
Spencer Limits, EE Washout
These computers have approximately the same M-value as the RDP
During a surface interval, all compartments washout/release nitrogen at their underwater halftime rate (EE = Exponential uptake, Exponential release).
This means the very fast compartments, which control deeper dives, will completely unload all their nitrogen, permitting dive profiles beyond what has been demonstrated to work reliably
e.g. three 10 minute dives to 40metres with only 30 minutes surface interval between them.
Spencer Limits, 60 Minute Washout
The computers draw upon data used testing the RDP.
At the surface, all compartments with 60minute or faster halftimes, washout at the 60minute rate, slower compartments washout at their underwater halftime rate, like the RDP.
Dives are very similar to the RDP.
Buhlmann Limits, EE Washout
These computers use lower M-values based on the work of Dr Buhlmann.
Compartments release nitrogen/washout at their underwater halftime rate.
These computers permit shorter single dives than the RDP.
However, due to lower M-values, they permit repetitive dives similar to the RDP, although they may still allow repetitive deep profiles with short surface intervals beyond what has been shown to work reliably.
PADI RDP
Surface interval M-values
Spencer Limits/ Not the same Similar
EE Washout So allows deep dives after a short surface interval
Spencer Limits/ Similar Similar
60 Min Washout So 5 minute compartment washes out at 60 minutes,
Anything over 60 minutes washes out at its
Underwater rate
Buhlmann Limits/ Not the same Not the same
EE Washout More conservative
Divers should not share computers!
Computers and tables have the same theoretical basis, computers are not better or safer than tables
Follow all manufacturing recommendations on the decompression theory of the dive computer
End the dive based on the most conservative computer
Diving at Altitude
The PADI RDP, other tables and dive computer models assume surfacing at sea level, and so diving at altitudes above 300m require special dive decompression procedures because there’s less atmospheric pressure at the surface, which affects dive table and dive computer calculations i.e. the ambient atmospheric pressure at altitude is less than at sea level.
At altitudes higher than 300m, the reduced atmospheric pressure when surfacing could make the tissue pressure gradient (the difference between the pressure of the nitrogen dissolved in the tissues and the surrounding/ambient pressure) too high, raising the risk of DCS.
According to dive decompression theory Actual depths must be converted to theoretical depths to find no decompression limits on the RDP. To use theoretical depth tables you know the altitude of the dive, and the special procedures include converting the actual depths to theoretical depths. A slower ascent rate of 9m per minute is used when diving at altitude.
Nitrogen narcosis may occur at shallower depths when diving at altitude!
Please learn more about other PADI IDC and Divemaster dive theory here:
